Goniobasis or Elimia catenaria dislocata
> Habitat & Distribution
Populations of P. catenaria bearing shells without spiral cords seem to be restricted to an unusual habitat: streams and small rivers of high groundwater content and good flow in the Piedmont and upper Coastal Plain ecoregions. Like typical P. catenaria catenaria, populations of P. catenaria dislocata are not found in acidic waters, and seem positively associated with limestone or marl outcroppings. But dislocata populations do not seem to be as strict in their requirement of rocky substrate, and can be found on bottoms of firm sand and woody debris. The distribution of P. catenaria dislocata is spotty, being recorded from several eastern North Carolina counties and Greensville County, Va, by Goodrich (1942) as well as from scattered South Carolina counties by Dillon & Keferl (2000) and tributaries of the Ogeechee River in Georgia. Its FWGNA incidence rank (calculated together with the typical P. catenaria catenaria) is I-5.
> Ecology & Life History
Although I am unaware of any ecological study directed specifically toward P. catenaria dislocata, I imagine the outline of its life history is similar to that of P. catenaria catenaria. Dislocata populations may reach high densities in smaller streams (e.g., cave springs flowing east into Lake Marion) and hence might be more likely to impact energy flow than typical catenaria.
It seems reasonable to expect that two years will be required for maturity, and that several years of iteroparous reproduction can be expected thereafter, as is the case for pleurocerids generally (Dazo 1965). This is life cycle Hi of Dillon (2000: 156 - 162).
> Taxonomy & Systematics
Ravenel and Reeve probably ought to share joint credit for describing "Melania" dislocata in the 19th century, but Ravenel (1834) is usually accorded the honor, by virtue of his earlier publication date. In either case, Goodrich lowered the nomen to subspecific rank under 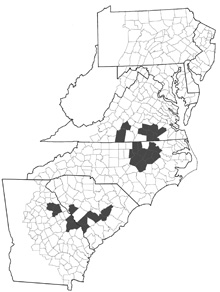 catenaria in 1942, a judgement wisely followed by Burch. The promotion of dislocata back to the full species level by Turgeon et al. (1998) was neither justified nor justifiable. See my essay of 5Mar14 from the link below for the complete taxonomic history.
catenaria in 1942, a judgement wisely followed by Burch. The promotion of dislocata back to the full species level by Turgeon et al. (1998) was neither justified nor justifiable. See my essay of 5Mar14 from the link below for the complete taxonomic history.
Superficially, populations of this subspecies are sometimes distinguishable from P. proxima only by the presence of faint costae on the apical whorls. But allozyme frequencies confirm a close genetic similarity to P. catenaria catenaria (Dillon & Reed 2002). The striking reduction in shell sculpture typically displayed by dislocata is likely a phenotypically-plastic response to soft substrate or some other peculiar aspect of their environment, as has been documented in Japanese Semisulcospira populations by Urabe (1998, 2000). And indeed, some populations inhabiting the Tar/Pamlico and Chowan drainages bear shells with sculpture intermediate between P. catenaria dislocata and typical P. catenaria catenaria, rendering the distinction quite subjective.
The diploid number is 2N = 34 (Dillon 1991). Mitochondrial CO1 and 16S sequence data are available in Dillon & Frankis (2004).
This species has travelled through three genera in thirty years. Although predominantly assigned to Goniobasis through most of the 20th century, in the 1980s many workers began placing it in the resurrected generic nomen, "Elimia." Both Goniobasis and Elimia were subsumed under Pleurocera by Dillon (2011). See my essay of 23Mar11 from the link below for more.
> Maps and Supplementary Resources
- Pleurocera catenaria distribution in Atlantic drainages (2023)
- Pleurocera distribution in Georgia and the Florida panhandle (2025)
- Virginia species account with county distribution (2011)
> Essays
- Taxonomic controversy has surrounded the generic nomina Pleurocera, Goniobasis, and Elimia for many years. The best entry into the subject would be my essay of 23Mar11, entitled Goodbye Goniobasis, Farewell Elimia. Links are available from that essay to older resources.
- See my FWGNA post of 16Mar09, The Snails The Dinosaurs Saw, for more on the genetics, taxonomy, and distribution of P. catenaria.
- The biological relationship between Pleurocera catenaria catenaria and P. catenaria dislocata served as the primary example in my essay of 4Feb14, What Is a Subspecies? That post also featured a figure comparing three shells from a Tar River population where the subspecies distinctions intergrade.
- The tortuous taxonomic history of the nomen "dislocata" also served as a primary example in my essay of 5Mar14, What Subspecies Are Not.
- Pleurocera catenaria dislocata received passing mention in my essay of 14Mar17, Fred Thompson, Elizabeth Mihalcik, and the Pleurocerids of Georgia.
> References
Burch, J. B. (1989) North American Freshwater Snails. Malacological Publications, Hamburg, MI.
Dazo, B. C. (1965) The morphology and natural history of Pleurocera acuta and Goniobasis livescens (Gastropoda: Cerithiacea: Pleuroceridae). Malacologia 3: 1 - 80.
Dillon, R. T., Jr. (1989) Karyotypic evolution in pleurocerid snails: I. Genomic DNA estimated by flow cytometry. Malacologia, 31: 197-203.
Dillon, R. T., Jr. (1991) Karyotypic evolution in pleurocerid snails: II. Pleurocera, Goniobasis, and Juga. Malacologia, 33: 339-344.
Dillon, R. T., Jr. (2000) The Ecology of Freshwater Molluscs. Cambridge, Cambridge University Press. 509 pp.
Dillon, R. T., Jr. (2011) Robust shell phenotype is a local response to stream size in the genus Pleurocera (Rafinesque, 1818). Malacologia 53: 265-277.
Dillon, R. T., Jr. & Keferl, E. (2000) A survey of the pleurocerid gastropods of South Carolina. In Freshwater Mollusk Symposia Proceedings, Part II, eds. Tankersley, Warmolts, Watters, Armitage, Johnson & Butler, pp. 153 - 160. Columbus: Ohio Biological Survey.
Dillon, R., T, Jr., & R. C. Frankis (2004) High levels of mitochondrial DNA sequence divergence in isolated populations of the freshwater snail genus Goniobasis. Amer. Malac. Bull., 19: 69 - 77.
Dillon, R. T., Jr. & Reed, A. (2002) A survey of genetic variation at allozyme loci among Goniobasis populations inhabiting Atlantic drainages of the Carolinas. Malacologia 44: 23 - 31.
Dillon, R.T., Jr. & Robinson, J.D (2009) The snails the dinosaurs saw: Are the pleurocerid populations of the Older Appalachians a relict of the Paleozoic Era? J. N. Am. Benthol. Soc. 28: 1-11.
Dillon, R. T., Jr. & Robinson, J. D. (2011) The opposite of speciation: Genetic relationships among the populations of Pleurocera (Gastropoda: Pleuroceridae) in central Georgia. Am. Malac. Bull. 29: 1 - 10.
Goodrich, C. (1942) The Pleuroceridae of the Atlantic coastal plain. Occas. Pprs. Mus. Zool. Univ. Mich., 456, 1-6.
Turgeon, D. D. et al. (1998) Common and scientific names of mollusks, second edition. American Fisheries Society Sp. Publ. 26.
Urabe, M. (1998) Contribution of genetic and environmental factors to shell shape variation in the lotic snail Semisulcospira reiniana (Prosobranchia: Pleuroceridae). J. Moll. Stud., 64: 329-343.
Urabe, M. (2000) Phenotypic modulation by the substratum of shell sculpture in Semisulcospira reiniana (Prosobranchia: Pleuroceridae). J. Moll. Stud., 66: 53-60.








