In recent years a variety of ranking systems have been developed by which species can be prioritized for the purpose of conservation. The nonprofit environmental organization NatureServe, for example, prioritizes the worldwide biota globally (G-ranks), nationally (N-ranks) or regionally (S-ranks) into five categories: 1 = critically imperiled, 2 = imperiled, 3 = vulnerable, 4 = apparently secure, and 5 = secure. The International Union for the Conservation of Nature also uses five ranks: CR = critically endangered, EN = endangered, VU = vulnerable, NT = near threatened and LC = least concern. A system of four ranks was advocated in the spurious review of Johnson et al. (2013), E = endangered, T = threatened, V = vulnerable, and CS = currently stable. A review of the many methods by which species have been categorized according to perceived conservation concern has been offered by Munton (1987).
Although the appeal of such systems to the natural resource agencies charged with protection of potentially endangered species is undeniable, such concepts as "threat" or "peril" or "endangerment" are by their nature entirely subjective. And although some connection is almost certainly made between conservation status rank and rarity in the minds of natural resource managers, the relationship has never been formally explored, at least in the case of freshwater snails. So, in 2011 we first suggested a ranking system based on incidence, offered as a compliment to (if not necessarily as an objective substitute for) the subjective systems of conservation status ranking currently in vogue.
> Version history
FWGNA synthesis v1.0 was initially proposed and forcefully advocated in essays posted on the FWGNA blogs of 12Dec11, 9Jan12, and 19Mar12. It was published in March 2012 as "FWGNA Circular No. 1" [pdf]. The database at that juncture contained 8,864 records of 57 species and subspecies, covering southern Atlantic drainages only. The distribution of commonness and rarity for those 57 species seemed to fit a lognormal model. Thus, a parametric system of incidence rankings was suggested, the rarest 5% being ranked “F1,” the remainder being divided by standard deviations around the mean: F2, F3, F4, and F5.
FWGNA synthesis v2.0 was proposed in the FWGNA website expansion of October 2013 and developed in the FWGNA blog post of 9Dec13. The analysis at that juncture was of 11,471 records collected from the Atlantic drainages of Georgia to the New York line. Number-theoretical analysis (EstimateS, Colwell 2013) returned no evidence that any rare species might have been missed in the list of 67 species (combining subspecies) returned.
The distribution of commonness and rarity demonstrated by the 67-species fauna did not appear lognormally distributed, but rather bimodal, with a secondary peak of high-incidence species. Thus, we shifted to a nonparametric model inspired by the work of Gaston (1994), suggesting a quartile system of I-ranks (I for “incidence”), again setting aside the rarest 5% as I-1. We also introduced the concept of peripheral species at that time, with the related phenomena of pseudo-rarity and non-apparent rarity.
FWGNA synthesis v2.1 was announced in my blog post of 19Nov15 and went up on the FWGNA website that same day. The analytical methods remained identical to that of version 2.0, although the database was expanded to 12,211 records and 69 species. This was the version published in Volume 1 of the FWGNA series by Dillon et al (2019).
FWGNA synthesis
v3.0. All versions of the FWGNA synthesis from
2012 – 2019 focused exclusively on the fauna of the Atlantic
drainages. On 19June19
we expanded our analysis to include both the fauna of the Tennessee
drainage above the Alabama line and the fauna of The Ohio above the
mouth of the Tennessee/Cumberland, a total of 19,643 records.
We
tallied 102 freshwater gastropod species in that expanded region
(lumping 9 additional subspecies within), set aside 6 (mostly
stygobiontic) species as being inadequately sampled to rank, and ranked
the remaining 96 by quartiles.
> Version 3.1
On 12May22 we announced the release of FWGNA Synthesis v3.1, an incremental (but not negligible) increase in our coverage beyond East Tennessee to include the entire freshwater gastropod fauna of the Tennessee/Cumberland drainage system, including North Alabama, Southern Kentucky, Middle Tennessee, and chips from the corners of Georgia and Mississippi. The FWGTN database increased from 1,798 records to 4,003. We also added 117 records to our FWGO database, collected since 2019, and 79 records to our older Atlantic databases, bringing the total records analyzed to 22,044.
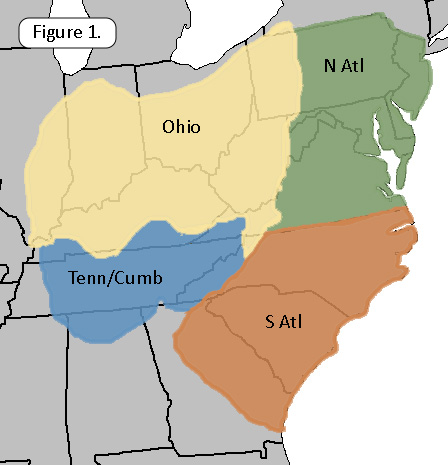
Lumping the 21 subspecific nomina under their parent species, the 107 species of freshwater gastropods we have documented from our vast 17-state study region are listed in Table Sy1. The number of discrete sites at which each species was observed, its “incidence,” is tabulated by subregion: The Ohio drainage, the Tennessee / Cumberland drainage, Atlantic drainages from Virginia to the New York line (N Atl) and Atlantic drainages of Georgia and the Carolinas (S Atl). A similar table, more simply formated, can be downloaded as an excel spreadsheet here [Table By1].
The ecological requirements of this fauna are exceptionally diverse. Seven of the species are stygobionts – obligately adapted to the cave environment – Antroselates spiralis, Holsingeria unthanksensis, Fontigens tartarea, F. turritella, and three Fontigens species as yet undescribed. Fontigens cryptica is apparently an obligate inhabitant of subterranean interstitial spaces, although so rarely collected alive that its biology is unknown. It seems unlikely to us that the data presented in Table Sy1 accurately reflect the abundance of these eight species.
Ecologically, Pomatiopsis lapidaria is a land snail. Essentially all 62 P. lapidaria records in our database have come from museums, primarily in collections from forest litter. As was the case with the eight subterranean species, it seems likely to us that our sampling methodology significantly underestimates the true abundance of P. lapidaria. It seems advisable therefore to exclude these nine species from further analysis, leaving 107 – 9 = 98 species eligible for ranking.
In Table Sy1 we have ordered the 98 freshwater gastropod species inhabiting our 17-state study area by their overall incidence in the FWGNA database. Unsurprisingly, the most common species would appear to be Physa acuta, with 3,467 records. Physa acuta is a cosmopolitan invasive, native to North America but spread to five other continents (Dillon et al. 2002) and was the most common species in all four of the regions as well as in the total. The 1,606 records of the next-most common species, Campeloma decisum, amount to less than half of the Physa records. Interestingly, Campeloma retained its second-place rank only in the South Atlantic drainages. In the other three subregions, pride of second place went to a pleurocerid: Leptoxis carinata in the North Atlantic drainages, Pleurocera semicarinata in the Ohio, and Pleurocera simplex in the Tennessee/Cumberland.
In
striking contrast, five species were represented in the freshwater
gastropod fauna of our 17-state region by but a single record, even
setting aside the nine species we consider undersampled.
Another
five species were represented by just two records, and another five by
just three.
> Rarity
Rabinowitz (1981) has famously pointed out that there are seven forms of rarity. We have no data on population sizes, however, nor any rigorous measure of habitat specificity for our 107 freshwater gastropod species. Our analysis here has focused entirely upon geographical distributions. The species found in the most spots are common by our definition, and those in the fewest spots are rare. This may be termed “incidence rarity.”
Convention would dictate that some special consideration might be extended to the extreme 5% of any distribution, normally distributed or otherwise. Thus, we suggest that the 5% of the species demonstrating the lowest number of incidences in a biota under consideration be assigned the rank “I-1,” by analogy to the “G1” rank of NatureServe. The “I” prefix here designates “incidence,” to emphasize that the present ranking system is based on incidence data, rather than subjective impressions of “global imperilment.”
Gaston (1994) has offered an admirable review of the term “rare” in all its various origins and biological usages. On the basis of clarity, versatility, consistency, and ease of use, he has suggested that the term "rare" be defined as “the first quartile of the frequency distribution of species abundances.” So because we have just set aside the 5% of species with the lowest incidence frequency as I-1, perhaps the 20% of the species remaining in the lowest quartile ought to be designated I-2. Then a straightforward application of Gaston’s system would suggest that the second quartile (between the rarest set and the median) be designated I-3, and the third quartile I-4, and the fourth (most common) quartile designated I-5.
Then setting aside the terrestrial Pomatiopsis lapidaria and the eight subterranean species, the rightmost (“FWGNA”) column of Table Sy1 shows the vector of I-ranks resulting from an application of Gaston’s system to the 107 – 9 = 98 freshwater gastropod species inhabiting our 17-state study area.
> Peripheral Species
Murray et al. (1999) reported that a remarkable 91% of the
species
in the “tail” of a rank-abundance curve generated from the
canopy-forming vegetation of the Australian dry sclerophyll woodland
were significantly more abundant elsewhere. Murray and
colleagues
called these 91% the “somewhere-abundant” species, to distinguish that
group from the 9% that were “everywhere-sparse.”
Similar
phenomena have been noted in many animal communities. Gaston
(1994) has reported that the vast majority of the British bird species
demonstrating incidence rarity are “vagrants,” which he defines as “not
permanent members of the assemblage, do not breed, or do not have
self-sustaining populations.” Gaston notes that other terms
that
have been applied to describe such species include accidentals,
casuals, immigrants, incidentals, strays, tourists and
tramps.
Magurran & Henderson (2003) have added the term “occasionals”
for
rare species in estuarine fish communities. The literature of
plant community ecology includes the terms "peripheral" and "waif."
Similar
to the situation described by Murray and colleagues, the data in Table
Sy1 suggest to us two categories of rare species, the
“somewhere-abundant” and the “everywhere-sparse.” But we are
not
aware of any term in malacology analogous to “vagrant” or “occasional,”
probably because such terms imply greater dispersal capability than is
ordinarily assumed for freshwater mollusks.
We therefore
suggest adopting the botanical term “peripheral” for use in mollusk
community ecology to describe the situation where a rare species is
“somewhere-else-abundant.” We formally define a peripheral
species as demonstrating less than median incidence in a
region under
study, but greater than median incidence elsewhere. And we
suggest that all non-peripheral species in a study region be called
“core”
species.
Although there are few rigorous estimates of
the relative incidence of freshwater gastropod species outside the
present analysis, our reading of the malacological literature suggests
to us that 19 of the 48 species listed below the median in Table Sy1
probably demonstrate above-median incidence
elsewhere.
Five of these 49 are exotic invasives – Bithynia tentaculata, Potamopyrgus antipodarium, Pyrgophorus parvulus, Melanoides tuberculata and Pomacea maculata. The excellent New York survey of Jokinen (1992) and the Canadian survey of Clarke (1981) suggest to us that the following 11 species are more common to the north and west of our study area: Valvata tricarinata, Marstonia lustrica, Probythinella emarginata, Cincinnatia integra, Helisoma campanulata, Lymnaea catascopium, L. stagnalis, L. caperata, Gyraulus deflectus, G. circumstriatus and Aplexa elongata. And the Florida survey of Thompson (1999) suggests that the following 7 species are more common further south: Pomacea paludosa, Pleurocera floridensis, Floridobia floridana, Lymnaea cubensis/viator, Helisoma scalare, Biomphalaria havanensis, and Hebetancylus excentricus. A lower-case “p” has been appended to the incidence ranks of all 23 of these species in Table Sy1, to indicate their (hypothesized) peripheral status in our 17-state study region.
> Pseudo-rarity and Non-apparent rarity
The 8 core species marked I-1 and the 6 core species marked I-2 in the rightmost column of Table Sy1 are clearly rare, demonstrating incidences in the bottom quartile. Gaston (1994) coined two terms relevant to the situation regarding the other 25 – 14 = 11 species in the first quartile of Table Sy1, however, "pseudo-rarity" and "non-apparent rarity." The former term would describe the 7 species we have listed as I-1p and the 4 species we have listed as I-2p, because although their incidence ranks them in the bottom quartile of the 98 species in our study area, there is reason to think that they are not rare elsewhere. Indeed, those 11 pseudo-rare species have displaced 11 species that should have occupied their spots in the bottom quartile. Thus the 11 core species marked I-3* in the rightmost column of Table Sy1 (without the modifier "p") all demonstrate non-apparent rarity. They are legitimately rare, and deserved to have appeared in the bottom quartile, but their rarity was obscured by the 11 pseudo-rare, peripheral species.
> Future Prospects
Note that (at least) 9 of the species listed in Table Sy1 have been
considered “invasive,” demonstrating a potential for rapid range
expansion in historic times: Cipangopaludina
japonica, C. chinensis, Viviparus
georgianus, and V.
subpurpureus, and the five species listed two
paragraphs above. At least two other species may have
obtained
representation in Table Sy1 by artificial introduction (Pomacea paludosa
and Biomphalaria
havanensis), although demonstrating little potential
to spread. We have elected not to treat these species
differently. The four viviparids listed above have now spread
to
above-median incidence in our 17-state study area and are hence
considered “core” species by our definition. Some of the
other
species may ultimately transfer from peripheral to core status, as
well. We do not expect the designations in the far rightmost
column of Table Sy1 to remain static.
Indeed, we expect the
opposite. In April of 2024 the FWGNA survey expanded to include
the Great Plains states of Kansas, Nebraska, South Dakota and North
Dakota [FWGGP].
We have not integrated those data into our continental
synthesis as yet, waiting on results from Missouri and Arkansas, but
when we do, the effect on Incidence Rankings will be significant.
Our mid-range plans also anticipate expansion into Florida
and the Gulf drainages of Georgia. The I-ranks currently shown in
Table Sy1 should be
considered
subject to revision for quite a few years to come.
> Essays
- Our initial effort to develop a (parametric) theory of commonness and rarity for freshwater gastropods was based on the incidence of 57 species in four states only. This analysis was introduced and justified in my blog posts of 12Dec11 and 9Jan12: "Toward the Scientific Ranking of Conservation Status."
- I introduced the first nine-state, nonparametric version of this analysis in my blog post of 9Dec13, "What is Rarity?" That particular essay focused on Gaston's quartile definition. The 2013 FWGNA database at that juncture contained 11,471 records from the Atlantic drainages, representing 67 species.
- I focused on the subjects of "peripheral" species, pseudo-rarity and non-apparent rarity in my follow-up essay of 6Jan14, "Why is Rarity?"
- Version 2.1 of the FWGNA synthesis (12,211 records, 69 species) was an incremental (although not negligible) expansion of our 2013 analysis. It was announced in my blog post of 19Nov15.
- I announced Version 3.0 of the FWGNA synthesis on 19June19, expanding coverage west over the Blue Ridge to include drainages of The Ohio and East Tennessee, bringing the total records to 19,643 and the total species to 102.
>
References
Clarke, A. H. 1981. The
Freshwater Molluscs of Canada. National Museum of Natural
Sciences, National Museums of Canada, Ottawa.
Colewell, R. K. 2013.
EstimateS: Statistical estimation of species richness and shared
species from samples. Version 9. User's Guide and application published
at: http://purl.oclc.org/estimates.
Dillon, R.T., Jr., M.J.
Ashton, W.K. Reeves, T.P. Smith, T.W. Stewart, & B.T. Watson
(2019)
Atlantic drainages, Georgia through Pennsylvania. Freshwater
Gastropods of North America, Volume 1. FWGNA Press.
199 pp. [html]
Dillon, R. T., A. R.
Wethington, J. M. Rhett and T. P. Smith. (2002) Populations
of the European freshwater pulmonate Physa acuta are not
reproductively isolated from American Physa heterostropha
or Physa integra.
Invertebrate Biology 121: 226-234. [PDF]
Gaston, K.
J. 1994. Rarity. Chapman
& Hall, London. 205 pp.
Johnson, P.D. Bogan,
Brown,
Burkhead, Cordeiro, Garner, Hartfield, Lepitzki, Mackie, Pip, Tarpley,
Tiemann, Whelan & Strong 2013.
Conservation status of freshwater gastropods of Canada and the United
States. Fisheries 38: 247- 282.
Jokinen, E.
H. 1992. The Freshwater Snails
(Mollusca: Gastropoda) of New York State. Albany: New York
State Museum. 112 pp.
Munton,
P. 1987. Concepts of threat
to the survival of species used in Red Data books and similar
compilations. Pp 72- 95 In The Road to Extinction (R. Fitter
& M. Fitter, eds.) IUCN/UNEP. Gland,
Switzerland
Magurran, A. E. &
P. A. Henderson 2003. Explaining the
excess of rare species in natural species abundance
distributions. Nature 422: 714-716.
Murray, B. R.,
B. L. Rice, D. A. Keith, P. J. Myerscough, J. Howell, A. G. Floyd, K.
Mills & M. Westoby 1999.
Species in the tail of rank-abundance curves. Ecology 80:
1806-1816.
Rabinowitz,
D. 1981. Seven forms of
rarity. Pp 205 – 217 in The Biological Aspects of Rare Plant
Conservation (H. Synge, ed.) Wiley, NY.
Thompson, F.
G. 1999. An identification manual for
the freshwater snails of Florida. Walkerana 10 (23): 1 – 96.